Design, develop and licence medicines specifically for children.
Meeting an unmet need
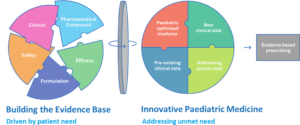
Currently, over half of the medicines children take are in the wrong format and/or are not licensed for use in children.
At Proveca we are trying to address this current unmet need in paediatric medicines by:
- Identifying medicines that are not licensed for use in children and require a paediatric licence (new indication) and/or require an improved administration format, to address an unmet need.
- Working alongside clinicians, parents, carers, and children, we are able to tailor the features of our medicines to meet the specific requirements of children.
- Build the evidence base through clinical studies, data generation and pharmaceutical development.
- Have strong internal expertise in formulation feasibility, technical development, clinical programmes, and regulatory pathways including the PUMA pathway.
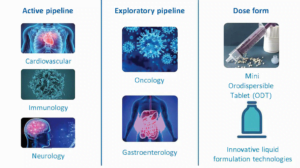
Key therapeutic areas & active pipeline
At Proveca we design, develop and license medicines for children, with a core focus in neurology, cardiology and immunology. We identify products which require a new paediatric license (new indication) and/or an improved format for administration.
Research & science collaborations
Proveca’s Research & Science strategy is to work with leading children’s hospitals, academic groups, and paediatric networks across Europe to identify unmet medical needs and collaborate on research to help with our mission – to improve medicines for children.
Our research focus has the following themes:
Active Collaborations and Associated Paediatric Networks:
- Alder Hey Children’s NHS Foundation Trust
- Manchester University NHS Foundation Trust
- Aston University, Birmingham UK
- Royal College of Surgeons Ireland
- Neonatal Paediatric Pharmacy Group
- EuPFI
- ESDPPP
- EFGCP
- Liverpool John Moores University
- Connect 4 Children
Alder Hey Children’s NHS Foundation Trust
Alder Hey Children’s Hospital and Proveca Ltd joined forces in January 2022 with the aim of improving medicines for children and young people.
Click Here to see our press release for further information.
Aston University
Proveca and Aston University Collaboration – Student projects
Following the success of the joint PhD project and other R&D projects including formulation development of needed paediatric products, Proveca and Aston University have formed a partnership to continue working together.
Please click Here to see our press release for further information.
Publications:
- 2021 Development of an Age-Appropriate Mini Orally Disintegrating Carvedilol Tablet with Paediatric Biopharmaceutical Considerations
- 2022 Paediatric specific dosage forms: Patient and formulation considerations
- 2023 Virtual Clinical Trials Guided Design of an Age-Appropriate Formulation and Dosing Strategy of Nifedipine for Paediatric Use
Summer Students Programme
Each year we invite applications for two student placements (undergraduate or postgraduate students).
Dates: The placements will be between June and July 2023
Location: Hybrid working, Central Manchester
Application deadline: Applications are accepted between the 1st of January – 28th of February annually
If you would like to enquire or apply for a student placement, please contact info@proveca.com
Student Testimonials:
Dedicated to improving children’s medicines
Lets work together to improve medicines for children:
Contact us at dream@proveca.com to help us identify and meet unmet paediatric needs








